Catinone sostituito


I catinoni sostituiti sono molecole ad azione stimolante ed entactogena-empatogena, derivati dal catinone.
Sono caratterizzati da un nucleo di fenetilammina con un gruppo alchilico attaccato al carbonio alfa e un gruppo chetone attaccato al carbonio beta, insieme a sostituzioni aggiuntive.[1][2][3][4][5] Il catinone si trova naturalmente nella pianta khat le cui foglie vengono masticate come droga ricreativa.[6]
Elenco dei catinoni sostituiti[modifica | modifica wikitesto]
I derivati possono essere prodotti mediante sostituzioni in quattro posizioni della molecola del catinone:
- R 1 = idrogeno o qualsiasi combinazione di uno o più sostituenti alchilici, alcossi, alchilendiossi, aloalchilici o alogenuri
- R 2 = idrogeno o qualsiasi gruppo alchilico
- R 3 = idrogeno, qualsiasi gruppo alchilico o incorporazione in una struttura ciclica
- R 4 = idrogeno, qualsiasi gruppo alchilico o incorporazione in una struttura ciclica
La tabella seguente mostra i derivati importanti che sono stati riportati:[7][8][9][10][11][12][13][14][15][16][17][18][19][20]
| Structure | Compound | R1 | R2 | R3 | R4 | CAS number |
|---|---|---|---|---|---|---|
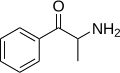
|
Cathinone | H | Me | H | H | 71031-15-7 |
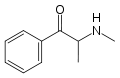
|
Methcathinone | H | Me | H | Me | 5650-44-2 |
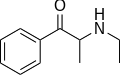
|
Ethcathinone | H | Me | H | Et | 51553-17-4 |

|
Propylcathinone | H | Me | H | nPr | 52597-14-5 |

|
Buphedrone | H | Et | H | Me | 408332-79-6 |
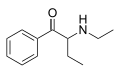
|
NEB | H | Et | H | Et | 1354631-28-9 |

|
N-methyl-N-ethylbuphedrone | H | Et | Me | Et | |

|
Pentedrone | H | nPr | H | Me | 879722-57-3 |

|
N-Ethylpentedrone | H | nPr | H | Et | 18268-16-1 |
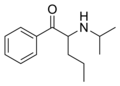
|
N-Isopropylpentedrone | H | nPr | H | iPr | |

|
Hexedrone | H | nBu | H | Me | |
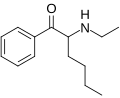
|
Ethyl-Hexedrone | H | nBu | H | Et | 18410-62-3 |

|
N-Butyl-hexedrone | H | nBu | H | nBu | |

|
N-Isobutylhexedrone (NDH) | H | nBu | H | i-Bu | |

|
Isohexedrone | H | iBu | H | Me | |

|
N-Ethylheptedrone | H | nPe | H | Et | |

|
Octedrone | H | hexyl | H | Me | |

|
Dimethylcathinone | H | Me | Me | Me | 15351-09-4 |
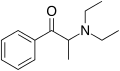
|
Diethylpropion | H | Me | Et | Et | 134-80-5 |

|
N-methyl-N-ethylcathinone | H | Me | Me | Et | 1157739-24-6 |
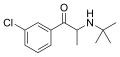
|
Bupropion (3-CBP) | 3-Cl | Me | H | t-Bu | 34911-55-2 |

|
Hydroxybupropion | 3-Cl | Me | H | 2-Me-3-OH-propan-2-yl | 357399-43-0 |

|
Mephedrone | 4-Me | Me | H | Me | 1189805-46-6 |

|
2-MMC | 2-Me | Me | H | Me | |

|
2-MEC | 2-Me | Me | H | Et | |

|
2-EEC | 2-Et | Me | H | Et | |

|
3-MMC | 3-Me | Me | H | Me | 1246816-62-5 |

|
3-MEC | 3-Me | Me | H | Et | |

|
3-EMC | 3-Et | Me | H | Me | |
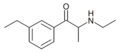
|
3-EEC | 3-Et | Me | H | Et | |
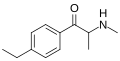
|
4-EMC | 4-Et | Me | H | Me | 1225622-14-9 |

|
4-EEC | 4-Et | Me | H | Et | |
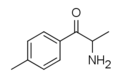
|
4-MC | 4-Me | Me | H | H | 31952-47-3 |

|
Benzedrone | 4-Me | Me | H | Bn | 1225617-75-3 |

|
2'-MeO-Benzedrone | 4-Me | Me | H | 2-MeO-Bn | |

|
4-MEC | 4-Me | Me | H | Et | 1225617-18-4 |

|
4-MPC | 4-Me | Me | H | nPr | |
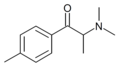
|
N,N-DMMC | 4-Me | Me | Me | Me | |

|
N,N-MEMC | 4-Me | Me | Me | Et | |

|
N,N-DEMC | 4-Me | Me | Et | Et | 676316-90-8 |

|
4-MEAP | 4-Me | Pr | H | Et | 746540-82-9 |

|
EDMC | 4-Et | Me | Me | Me | |
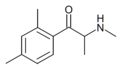
|
2,4-DMMC | 2,4-dimethyl | Me | H | Me | |

|
2,4-DMEC | 2,4-dimethyl | Me | H | Et | |
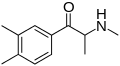
|
3,4-DMMC | 3,4-dimethyl | Me | H | Me | 1082110-00-6 |

|
3,4-DMEC | 3,4-dimethyl | Me | H | Et | |

|
2,4,5-TMMC | 2,4,5-trimethyl | Me | H | Me | |

|
2,4,5-TMOMC | 2,4,5-trimethoxy | Me | H | Me | |

|
3,4,5-TMOMC | 3,4,5-trimethoxy | Me | H | Me | |
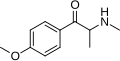
|
Methedrone | 4-MeO | Me | H | Me | 530-54-1 |

|
Dimethedrone | 4-MeO | Me | Me | Me | 91564-39-5 |

|
Ethedrone | 4-MeO | Me | H | Et | |

|
2-MOMC | 2-MeO | Me | H | Me | |

|
3-MOMC | 3-MeO | Me | H | Me | |
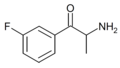
|
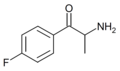
3-FC | 3-F | Me | H | H | |
|
4-FC | 4-F | Me | H | H | 80096-51-1 |

|
2-FMC | 2-F | Me | H | Me | 1186137-35-8 |

|
2-FEC | 2-F | Me | H | Et | |

|
3-FMC | 3-F | Me | H | Me | 1049677-77-1 |

|
3-FEC | 3-F | Me | H | Et | |

|
2-CMC | 2-Cl | Me | H | Me | |

|
2-BMC | 2-Br | Me | H | Me | |

|
2-IMC | 2-I | Me | H | Me | |

|
2-TFMAP | 2-CF3 | Me | H | Me | |

|
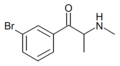
Clophedrone | 3-Cl | Me | H | Me | 1049677-59-9 |

|
3-CEC | 3-Cl | Me | H | Et | |
|
3-BMC | 3-Br | Me | H | Me | |

|
3-IMC | 3-I | Me | H | Me | |

|
3-TFMAP | 3-CF3 | Me | H | Me | |

|
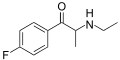
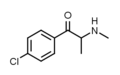
Flephedrone | 4-F | Me | H | Me | 447-40-5 |
|
4-FEC | 4-F | Me | H | Et | 1225625-74-0 |
|
Clephedrone | 4-Cl | Me | H | Me | 1225843-86-6 |

|
4-CEC | 4-Cl | Me | H | Et | |

|
4-CiPC | 4-Cl | Me | H | iPr | |
|
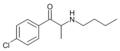
4-CBC | 4-Cl | Me | H | nBu | |

|
4-CDMC | 4-Cl | Me | Me | Me | |
|
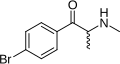
Brephedrone | 4-Br | Me | H | Me | 486459-03-4 |

|
4-BEC | 4-Br | Me | H | Et | |
|
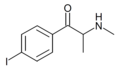
4-IMC | 4-I | Me | H | Me | |

|
4-TFMAP | 4-CF3 | Me | H | Me | |

|
4-EFMC | 4-(2-fluoroethyl) | Me | H | Me | |

|
4-MTMC | 4-SCH3 | Me | H | Me | |
|
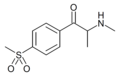
4-MSMC | 4-SO2CH3 | Me | H | Me | |

|
4-PHMC | 4-phenyl | Me | H | Me | |

|
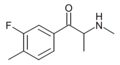
Mexedrone | 4-Me | methoxymethyl | H | Me | |
|
FMMC | 3-F-4-Me | Me | H | Me | |

|
MFMC | 3-Me-4-F | Me | H | Me | |

|
MMOMC | 3-Me-4-MeO | Me | H | Me | |
|
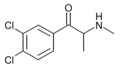
3,4-DCMC | 3,4-dichloro | Me | H | Me | |

|
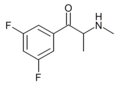
3,5-DCMC | 3,5-dichloro | Me | H | Me | |
|
3,5-DFMC | 3,5-difluoro | Me | H | Me | |

|
2,5-DMOMC | 2,5-dimethoxy | Me | H | Me | |

|
βk-2C-C | 2,5-dimethoxy-4-chloro | H | H | H | 1538191-15-9 |

|
βk-2C-B | 2,5-dimethoxy-4-bromo | H | H | H | 807631-09-0 |

|
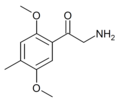
βk-2C-I | 2,5-dimethoxy-4-iodo | H | H | H | |
|
βk-2C-D | 2,5-dimethoxy-4-methyl | H | H | H | 1368627-25-1 |

|
βk-2C-E | 2,5-dimethoxy-4-ethyl | H | H | H | 1517021-02-1 |

|
βk-2C-P | 2,5-dimethoxy-4-propyl | H | H | H | |

|
βk-2C-iP | 2,5-dimethoxy-4-isopropyl | H | H | H | 1511033-62-7 |

|
βk-DOB | 2,5-dimethoxy-4-bromo | Me | H | H | |
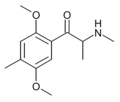
|
βk-MDOM | 2,5-dimethoxy-4-methyl | Me | H | Me | |

|
βk-MDA | 3,4-methylenedioxy | Me | H | H | 80535-73-5 |
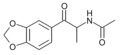
|
N-acetyl-βk-MDA | 3,4-methylenedioxy | Me | H | acetyl | |

|
2,3-MDMC | 2,3-methylenedioxy | Me | H | Me | |
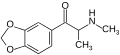
|
Methylone | 3,4-methylenedioxy | Me | H | Me | 186028-79-5 |

|
Dimethylone | 3,4-methylenedioxy | Me | Me | Me | |

|
N-acetyl-methylone | 3,4-methylenedioxy | Me | acetyl | Me | |

|
N-hydroxy-methylone | 3,4-methylenedioxy | Me | hydroxy | Me | |

|
Ethylone | 3,4-methylenedioxy | Me | H | Et | 1112937-64-0 |

|
Diethylone | 3,4-methylenedioxy | Me | Et | Et | |

|
N-acetyl-ethylone | 3,4-methylenedioxy | Me | acetyl | Et | |

|
N-isopropyl-βk-MDA | 3,4-methylenedioxy | Me | H | iPr | |
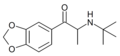
|
MDPT | 3,4-methylenedioxy | Me | H | t-Bu | |

|
BMDP | 3,4-methylenedioxy | Me | H | Bn | |
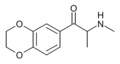
|
3,4-EDMC | 3,4-ethylenedioxy | Me | H | Me | |

|
βk-IMP | 3,4-trimethylene | Me | H | Me | |
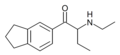
|
βk-IBP | 3,4-trimethylene | Et | H | Et | |

|
βk-IVP | 3,4-trimethylene | nPr | H | Et | |
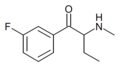
|
3-Fluorobuphedrone | 3-F | Et | H | Me | |

|
4-Fluorobuphedrone | 4-F | Et | H | Me | |
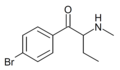
|
4-Bromobuphedrone | 4-Br | Et | H | Me | |

|
4-MeMABP | 4-Me | Et | H | Me | 1336911-98-8 |
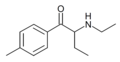
|
4-Me-NEB | 4-Me | Et | H | Et | |

|
4-F-NEB | 4-F | Et | H | Et | |

|
4-Me-DMB | 4-Me | Et | Me | Me | |

|
3,4-DMEB | 3,4-dimethyl | Et | H | Et | |
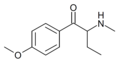
|
4-Methoxybuphedrone | 4-MeO | Et | H | Me | |

|
Butylone | 3,4-methylenedioxy | Et | H | Me | 802575-11-7 |

|
Eutylone | 3,4-methylenedioxy | Et | H | Et | 802855-66-9 |
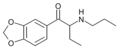
|
βk-PBDB | 3,4-methylenedioxy | Et | H | nPr | |
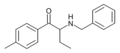
|
Bn-4-MeMABP | 4-Me | Et | H | Bn | 1445751-39-2 |

|
BMDB | 3,4-methylenedioxy | Et | H | Bn | 1445751-47-2 |

|
βk-DMBDB | 3,4-methylenedioxy | Et | Me | Me | 802286-83-5 |

|
βk-MMDMA | 3,4-methylenedioxy-5-MeO | Me | H | Me | |
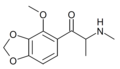
|
βk-MMDMA-2 | 2-MeO-3,4-methylenedioxy | Me | H | Me | |

|
βk-DMMDA | 2,5-diMeO-3,4-methylenedioxy | Me | H | H | |

|
5-Methylmethylone | 3,4-methylenedioxy-5-Me | Me | H | Me | 1364933-83-4 |

|
5-Methylethylone | 3,4-methylenedioxy-5-Me | Me | H | Et | 1364933-82-3 |

|
2-Methylbutylone | 2-Me-3,4-methylenedioxy | Et | H | Me | 1364933-86-7 |

|
5-Methylbutylone | 3,4-methylenedioxy-5-Me | Et | H | Me | 1354631-29-0 |

|
Pentylone | 3,4-methylenedioxy | nPr | H | Me | 698963-77-8 |

|
N-Ethylpentylone | 3,4-methylenedioxy | nPr | H | Et | 727641-67-0 |
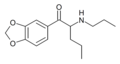
|
N-propylpentylone | 3,4-methylenedioxy | nPr | H | nPr | |
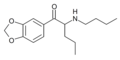
|
N-butylpentylone | 3,4-methylenedioxy | nPr | H | nBu | |

|
Dipentylone | 3,4-methylenedioxy | nPr | Me | Me | |

|
Hexylone | 3,4-methylenedioxy | nBu | H | Me | |

|
N-Ethylhexylone | 3,4-methylenedioxy | nBu | H | Et | |
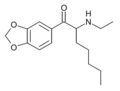
|
N-Ethylheptylone | 3,4-methylenedioxy | nPe | H | Et | |

|
4-MEAP | 4-Me | nPr | H | Et | 746540-82-9 |

|
3,4-DMEP | 3,4-dimethyl | nPr | H | Et | |
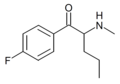
|
4-F-Pentedrone | 4-F | nPr | H | Me | |

|
4-Cl-Pentedrone | 4-Cl | nPr | H | Me | |
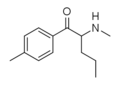
|
4-Methylpentedrone | 4-Me | nPr | H | Me | 1373918-61-6 |

|
DL-4662 | 3,4-dimethoxy | nPr | H | Et | 1674389-55-9 |

|
4-F-iPr-norpentedrone | 4-F | nPr | H | iPr | |

|
3-CBV | 3-Cl | nPr | H | tBu | |
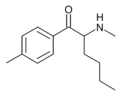
|
4-methylhexedrone | 4-Me | nBu | H | Me | |
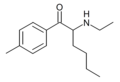
|
MEH | 4-Me | nBu | H | Et | |

|
4-F-hexedrone | 4-F | nBu | H | Me | |
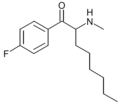
|
4-F-octedrone | 4-F | hexyl | H | Me | |
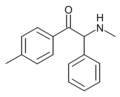
|
α-phenylmephedrone | 4-Me | phenyl | H | Me | |
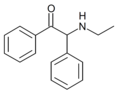
|
βk-Ephenidine | H | phenyl | H | Et | 22312-16-9 |
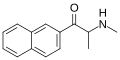
|
"NRG-3" | β-naphthyl instead of phenyl | Me | H | Me | |

|
βk-Methiopropamine | thiophen-2-yl instead of phenyl | Me | H | Me | 24065-17-6 |

|
βk-5-MAPB | benzofuran-5-yl instead of phenyl | Me | H | Me | |

|
βk-6-MAPB | benzofuran-6-yl instead of phenyl | Me | H | Me | |
|
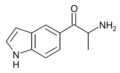
βk-5-IT | indol-5-yl instead of phenyl | Me | H | H | |

|
α-Phthalimidopropiophenone | H | Me | phthalimido | 19437-20-8 | |
|
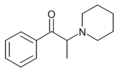
PPPO | H | Me | piperidinyl | ||

|
PPBO | H | Et | piperidinyl | ||

|
FPPVO | 4-F | nPr | piperidinyl | ||

|
MDPV-azepane | 3,4-methylenedioxy | nPr | azepane | ||

|
Caccure 907 | 4-SCH3 | α,α-di-Me | morpholinyl | ||
|
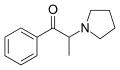
α-PPP | H | Me | pyrrolidinyl | 19134-50-0 | |

|
α-PBP | H | Et | pyrrolidinyl | 13415-54-8 | |

|
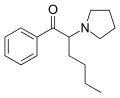
α-PVP (O-2387) | H | nPr | pyrrolidinyl | 14530-33-7 | |
|
α-PHP | H | nBu | pyrrolidinyl | 13415-86-6 | |

|
α-PHiP | H | iBu | pyrrolidinyl | ||

|
α-PEP (α-PHPP) | H | nPe | pyrrolidinyl | 13415-83-3 | |
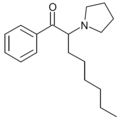
|
α-POP | H | hexyl | pyrrolidinyl | ||
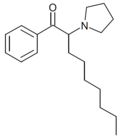
|
α-PNP | H | heptyl | pyrrolidinyl | ||

|
DPPE | H | phenyl | pyrrolidinyl | 27590-61-0 | |

|
α-PcPeP | H | cyclopentyl | pyrrolidinyl | ||

|
α-PCYP | H | cyclohexyl | pyrrolidinyl | 1803168-11-7 | |

|
2-MePPP | 2-Me | Me | pyrrolidinyl | ||

|
3-MePPP | 3-Me | Me | pyrrolidinyl | 1214940-01-8 | |

|
4-MePPP | 4-Me | Me | pyrrolidinyl | 1313393-58-6 | |
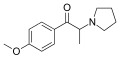
|
MOPPP | 4-MeO | Me | pyrrolidinyl | 478243-09-3 | |

|
3-F-PPP | 3-F | Me | pyrrolidinyl | 1214939-99-7 | |

|
FPPP | 4-F | Me | pyrrolidinyl | 28117-76-2 | |

|
Cl-PPP | 4-Cl | Me | pyrrolidinyl | ||
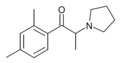
|
2,4-DMPPP | 2,4-dimethyl | Me | pyrrolidinyl | ||

|
3-MPBP | 3-Me | Et | pyrrolidinyl | 1373918-60-5 | |

|
3-F-PBP | 3-F | Et | pyrrolidinyl | 1373918-59-2 | |

|
MPBP | 4-Me | Et | pyrrolidinyl | 732180-91-5 | |

|
FPBP | 4-F | Et | pyrrolidinyl | 1373918-67-2 | |

|
EPBP | 4-Et | Et | pyrrolidinyl | ||
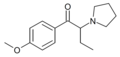
|
MOPBP | 4-MeO | Et | pyrrolidinyl | ||
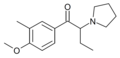
|
MMOPBP | 3-Me-4-MeO | Et | pyrrolidinyl | ||
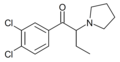
|
O-2384 | 3,4-dichloro | Et | pyrrolidinyl | 850352-65-7 | |

|
Pyrovalerone (O-2371) | 4-Me | nPr | pyrrolidinyl | 3563-49-3 | |

|
3-F-PVP | 3-F | nPr | pyrrolidinyl | ||
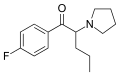
|
FPVP | 4-F | nPr | pyrrolidinyl | 850352-31-7 | |

|
4-Cl-PVP | 4-Cl | nPr | pyrrolidinyl | ||

|
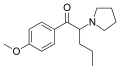
4-Br-PVP | 4-Br | nPr | pyrrolidinyl | ||
|
MOPVP | 4-MeO | nPr | pyrrolidinyl | 5537-19-9 | |

|
DMOPVP | 3,4-dimethoxy | nPr | pyrrolidinyl | 850442-84-1 | |

|
DMPVP | 3,4-dimethyl | nPr | pyrrolidinyl | ||

|
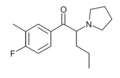
O-2390 | 3,4-dichloro | nPr | pyrrolidinyl | 850352-61-3 | |
|
MFPVP | 3-methyl-4-fluoro | nPr | pyrrolidinyl | ||

|
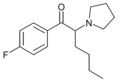
MPHP | 4-Me | nBu | pyrrolidinyl | 34138-58-4 | |
|
FPHP | 4-F | nBu | pyrrolidinyl | ||

|
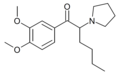
4-Cl-PHP | 4-Cl | nBu | pyrrolidinyl | ||
|
DMOPHP | 3,4-dimethoxy | nBu | pyrrolidinyl | ||
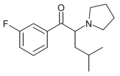
|
3F-PiHP | 3-F | iBu | pyrrolidinyl | ||

|
4F-PiHP | 4-F | iBu | pyrrolidinyl | ||
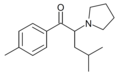
|
O-2394 | 4-Me | iBu | pyrrolidinyl | 850352-51-1 | |
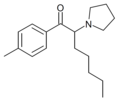
|
MPEP | 4-Me | pentyl | pyrrolidinyl | ||

|
4F-PV8 | 4-F | pentyl | pyrrolidinyl | ||
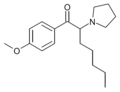
|
4-MeO-PV8 | 4-MeO | pentyl | pyrrolidinyl | ||
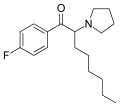
|
4F-PV9 | 4-F | hexyl | pyrrolidinyl | ||

|
4-MeO-PV9 | 4-MeO | hexyl | pyrrolidinyl | ||
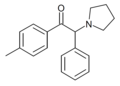
|
α-Phenylpyrovalerone | 4-Me | phenyl | pyrrolidinyl | ||

|
MDPPP | 3,4-methylenedioxy | Me | pyrrolidinyl | 783241-66-7 | |

|
MDMPP | 3,4-methylenedioxy | α,α-di-Me | pyrrolidinyl | ||

|
MDPBP | 3,4-methylenedioxy | Et | pyrrolidinyl | 784985-33-7 | |

|
MDPV | 3,4-methylenedioxy | nPr | pyrrolidinyl | 687603-66-3 | |
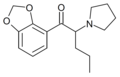
|
2,3-MDPV | 2,3-methylenedioxy | nPr | pyrrolidinyl | ||

|
5-Me-MDPV | 3,4-methylenedioxy-5-Me | nPr | pyrrolidinyl | ||
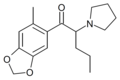
|
6-Me-MDPV | 2-Me-4,5-methylenedioxy | nPr | pyrrolidinyl | ||
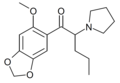
|
6-MeO-MDPV | 2-MeO-4,5-methylenedioxy | nPr | pyrrolidinyl | ||

|
Br-MeO-MDPV | 2,3-methylenedioxy-4-MeO-5-Br | nPr | pyrrolidinyl | ||
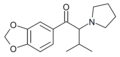
|
MDPiVP | 3,4-methylenedioxy | iPr | pyrrolidinyl | ||
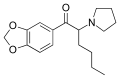
|
MDPHP | 3,4-methylenedioxy | nBu | pyrrolidinyl | 776994-64-0 | |

|
MDPEP (MD-PV8) | 3,4-methylenedioxy | pentyl | pyrrolidinyl | ||

|
MDPOP (MD-PV9) | 3,4-methylenedioxy | hexyl | pyrrolidinyl | ||
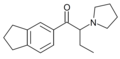
|
5-PPDI | 3,4-trimethylene | Et | pyrrolidinyl | ||

|
5-BPDI | 3,4-trimethylene | nPr | pyrrolidinyl | ||
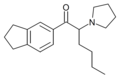
|
5-HPDI | 3,4-trimethylene | nBu | pyrrolidinyl | ||
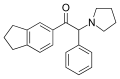
|
IPPV | 3,4-trimethylene | phenyl | pyrrolidinyl | ||
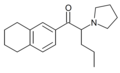
|
TH-PVP | 3,4-tetramethylene | nPr | pyrrolidinyl | ||

|
TH-PHP | 3,4-tetramethylene | nBu | pyrrolidinyl | ||

|
5-DBFPV | 2,3-dihydrobenzofuran-5-yl instead of Ph | nPr | pyrrolidinyl | 1620807-94-4 | |
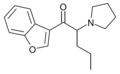
|
3-BF-PVP | benzofuran-3-yl instead of Ph | nPr | pyrrolidinyl | ||
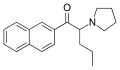
|
Naphyrone (O-2482) | β-naphthyl instead of phenyl | nPr | pyrrolidinyl | 850352-53-3 | |
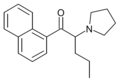
|
α-Naphyrone | α-naphthyl instead of phenyl | nPr | pyrrolidinyl | ||

|
α-PPT | thiophen-2-yl instead of phenyl | Me | pyrrolidinyl | ||

|
α-PBT | thiophen-2-yl instead of phenyl | Et | pyrrolidinyl | ||
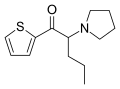
|
α-PVT | thiophen-2-yl instead of phenyl | nPr | pyrrolidinyl | 1400742-66-6 | |
Status legale[modifica | modifica wikitesto]
Il 2 aprile 2010, il Consiglio consultivo sull'abuso di droghe nel Regno Unito ha annunciato che sarebbe stato istituito un ampio divieto basato sulla struttura (e non quindi su una molecola precisa) di questa intera classe di composti, a seguito di un'ampia pubblicità sulle vendite sul mercato grigio e sull'uso ricreativo del mefedrone, un comune membro della famiglia. Questo divieto copre i composti con la suddetta struttura generale, con 28 composti specificatamente denominati.[21]

Le sostituzioni nella struttura generale per analoghi del nafirone soggetti al divieto possono essere descritte come segue:
- Cyc = qualsiasi sistema ad anello monociclico o fuso-policiclico (che non sia un anello fenilico o un sistema ad anello alchilendiossifenile), compresi gli analoghi in cui il sistema ad anello è sostituito in qualsiasi misura con sostituenti alchilici, alcossilici, aloalchilici o alogenuri, anche se non ulteriormente sostituiti in il sistema ad anello da uno o più altri sostituenti univalenti
- R 1 = idrogeno o qualsiasi gruppo alchile
- R 2 = idrogeno, qualsiasi gruppo alchilico o incorporazione in una struttura ciclica
- R 3 = idrogeno, qualsiasi gruppo alchilico o incorporazione in una struttura ciclica
Tuttavia, sono continuati ad apparire altri nuovi derivati, con il Regno Unito che ha segnalato più nuovi derivati del catinone rilevati nel 2010 rispetto a qualsiasi altro paese in Europa, con la maggior parte di essi identificati per la prima volta dopo l'entrata in vigore del divieto generico e quindi già illegali nonostante non siano mai stati segnalato in precedenza.[22]
Negli Stati Uniti, i catinoni sostituiti sono gli ingredienti psicoattivi dei "sali da bagno" che a partire dal luglio 2011 erano stati banditi da almeno 28 stati, ma non dal governo federale.[23]
Note[modifica | modifica wikitesto]
- ^ Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem. 2006 Feb 23;49(4):1420-32. PMID 16480278 DOI: 10.1021/jm050797a
- ^ Paillet-Loilier M, Cesbron A, Le Boisselier R, Bourgine J, Debruyne D. Emerging drugs of abuse: current perspectives on substituted cathinones. Subst Abuse Rehabil. 2014; 5: 37-52. PMID 24966713 DOI: 10.2147/SAR.S37257
- ^ Simmons SJ, Leyrer-Jackson JM, Oliver CF, Hicks C, Muschamp JW, Rawls SM, Olive MF. DARK Classics in Chemical Neuroscience: Cathinone-Derived Psychostimulants. ACS Chem. Neurosci. 2018; 9(10): 2379-2394. PMID 29714473 DOI: 10.1021/acschemneuro.8b00147
- ^ Beck O, Bäckberg M, Signell P, Helander A. Intoxications in the STRIDA project involving a panorama of psychostimulant pyrovalerone derivatives, MDPV copycats. Clin Toxicol (Phila). 2018 Apr;56(4):256-263. PMID 28895757 DOI: 10.1080/15563650.2017.1370097
- ^ Majchrzak M, Celiński R, Kuś P, Kowalska T, Sajewicz M. The newest cathinone derivatives as designer drugs: an analytical and toxicological review. Forensic Toxicol. 2018;36(1):33-50. PMID 29367861 DOI: 10.1007/s11419-017-0385-6
- ^ Colzato LS, Ruiz MJ, van den Wildenberg WP, Hommel B. Khat use is associated with impaired working memory and cognitive flexibility. PLoS One. 2011;6(6):e20602. PMID 21698275. accesso 2011-07-17.
- ^ Europol 2008 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2009 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2010 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2011 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2012 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2013 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2014 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2015 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2016 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Europol 2017 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ Maurer HH, Kraemer T, Springer D, Staack RF. Chemistry, pharmacology, toxicology, and hepatic metabolism of designer drugs of the amphetamine (ecstasy), piperazine, and pyrrolidinophenone types: a synopsis. Ther Drug Monit. 2004 Apr;26(2):127-31. PMID 15228152
- ^ Davis S, Rands-Trevor K, Boyd S, Edirisinghe M. The characterisation of two halogenated cathinone analogues: 3,5-difluoromethcathinone and 3,5-dichloromethcathinone. Forensic Sci Int. 2012 Apr 10;217(1-3):139-45. PMID 22088945 DOI: 10.1016/j.forsciint.2011.10.042
- ^ Błażewicz, A., Bednarek, E., Popławska, M. et al. Identification and structural characterization of synthetic cathinones: N-propylcathinone, 2,4-dimethylmethcathinone, 2,4-dimethylethcathinone, 2,4-dimethyl-α-pyrrolidinopropiophenone, 4-bromo-α-pyrrolidinopropiophenone, 1-(2,3-dihydro-1H-inden-5-yl)-2-(pyrrolidin-1-yl)hexan-1-one and 2,4-dimethylisocathinone. Forensic Toxicol 2019; 37: 288-307. DOI: 10.1007/s11419-018-00463-w
- ^ Westphal F, Girreser U, Angerer V, Auwärter V. Analytische Daten neuer 2-aminosubstituierter Methylendioxyvalerophenonderivate. Toxichem Krimtech, 2016 Jan 1; 83(1): 3–29.
- ^ Advisory Council on the Misuse of Drugs (UK). Consideration of the cathinones. 31 March 2010., su homeoffice.gov.uk. URL consultato il 17 luglio 2011. (archiviato dall'url originale il 22 settembre 2011).
- ^ European Monitoring Centre on Drugs and Drug Addiction. EMCDDA–Europol 2010 Annual Report on the implementation of Council Decision 2005/387/JHA. Archiviato il 14 marzo 2012 in Internet Archive. accesso 2011-07-17.
- ^ Goodnough A, Zezima K. An Alarming New Stimulant, Legal in Many States. New York Times 2011 July 16. accesso 2011-07-17.
Altri progetti[modifica | modifica wikitesto]
 Wikimedia Commons contiene immagini o altri file su Catinone sostituito
Wikimedia Commons contiene immagini o altri file su Catinone sostituito