Utente:Grasso Luigi/sanbox1/cloruro di solfenile
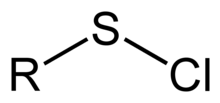
In organosulfur chemistry, a sulfenyl chloride is a functional group with the connectivity Template:Chem2, where R is alkyl[1] or aryl. Sulfenyl chlorides are reactive compounds that behave as sources of Template:Chem2. They are used in the formation of Template:Chem2 and Template:Chem2 bonds. According to IUPAC nomenclature they are named as alkyl thiohypochlorites, i.e. esters of thiohypochlorous acid.
Preparazione[modifica | modifica wikitesto]

Sulfenyl chlorides are typically prepared by chlorination of disulfides:[2][3]
This reaction is sometimes called the Zincke disulfide reaction, in recognition of Theodor Zincke.[4][5] Typically, sulfenyl halides are stabilized by electronegative substituents. This trend is illustrated by the stability of Template:Chem2 obtained by chlorination of carbon disulfide.
Some thioethers (Template:Chem2) with electron-withdrawing substituents undergo chlorinolysis of a Template:Chem2 bond to afford the sulfenyl chloride.[6][7]
Reactions[modifica | modifica wikitesto]
Perchloromethyl mercaptan (Template:Chem2) reacts with Template:Chem2 bonds in the presence of base to give the sulfenamides:
This method is used in the production of the fungicides Captan and Folpet.
Sulfenyl chlorides add across alkenes, for example ethylene:[8]
They undergo chlorination to the trichlorides:[3]
Sulfenyl chlorides react with water and alcohols to give sulfenyl esters (Template:Chem2):[9]
Route to sulfinyl halides[modifica | modifica wikitesto]
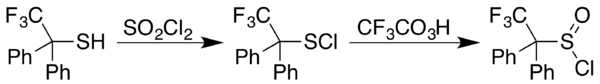
Sulfenyl chlorides can be converted to sulfinyl chlorides (RS(O)Cl). In one approach, the sulfinyl chloride is generated in two steps starting with reaction of a thiol (Template:Chem2) with sulfuryl chloride (Template:Chem2). In some cases the sulfenyl chloride results instead, as happens with 2,2,2-trifluoro-1,1-diphenylethanethiol. A trifluoroperacetic acid oxidation then provides a general approach to formation of sulfinyl chlorides from sulfenyl chlorides:[10]
Related compounds[modifica | modifica wikitesto]
Sulfenyl fluorides and bromides are also known.[11] Simple sulfenyl iodides are unknown because they are unstable with respect to the disulfide and iodine:
Sulfenyl iodides can be isolated as stable compounds if they bear alkyl steric protecting groups as part of a cavity-shaped framework, illustrating the technique of kinetic stabilization of a reactive functionality, as in the case of sulfenic acids.[12]
A related class of compounds are the alkylsulfur trichlorides, as exemplified by methylsulfur trichloride, Template:Chem2.[13]
The corresponding selenenyl halides, Template:Chem2, are more commonly encountered in the laboratory. Sulfenyl chlorides are used in the production of agents used in the vulcanization of rubber.
Note[modifica | modifica wikitesto]
- ^ Alkanesulfenyl Halides, 2008, pp. 544–550, ISBN 9781588905307.
- ^ (EN) Organic Syntheses, http://www.orgsyn.org/demo.aspx?prep=CV2P0455.
- ^ a b (EN) Organic Syntheses, http://www.orgsyn.org/demo.aspx?prep=CV5P0709.
- ^ (DE) Th. Zincke, Über eine neue Reihe aromatischer Schwefelverbindungen, in Chemische Berichte, vol. 44, n. 1, 1911, pp. 769–771, DOI:10.1002/cber.191104401109.
- ^ (DE) Über o-Nitrophenylschwefelchlorid und Umwandlungsprodukte, in Justus Liebig's Annalen der Chemie, vol. 391, n. 1, 1912, pp. 57–88, DOI:10.1002/jlac.19123910106.
- ^ F. B. Wells, C. F. H. Allen, 2,4-Dinitroaniline, in Organic Syntheses, vol. 15, 1935, DOI:10.15227/orgsyn.015.0022.
- ^ Norman Kharasch, Robert B. Langford, 2,4-Dinitrobenzenesulfenyl Chloride, in Organic Syntheses, vol. 44, 1964, DOI:10.15227/orgsyn.044.0047.
- ^ Brintzinger, H.; Langheck, M., "Synthesen mit Alkylschwefelchloriden (X. Mitteil. über organische Schwefelchloride)", Chemische Berichte 1954, volume 87, 325-330. DOI: 10.1002/cber.19540870306
- ^ Phenylsulfenylation of Nonactivated Carbon Atom by Photolysiis of Alkyl Benzenesulfenated: Preparation of 2-Phenylthio-5-heptanol, in Organic Syntheses, vol. 81, 2005, DOI:10.15227/orgsyn.081.0244.
- ^ Alkyl Chalcogenides: Sulfur-based Functional Groups, in Synthesis: Carbon with One Heteroatom Attached by a Single Bond, Elsevier, 1995, 113–276, ISBN 9780080423234.
- ^ (EN) Organic Syntheses, http://www.orgsyn.org/demo.aspx?prep=CV9P0662.
- ^ Stable Sulfenyl Iodide Bearing a Primary Alkyl Steric Protection Group with a Cavity-shaped Framework, in Chemistry Letters, vol. 38, n. 12, 2009, pp. 1188–1189, DOI:10.1246/cl.2009.1188.
- ^ Alkylsulfur Trihalides, in Sci. Synth., vol. 39, 2008, pp. 187–188.



![{\displaystyle {\ce {R-SCl + Cl2 -> [R-SCl2]Cl}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/deaf1569a9b4e7730eaea75337aa67878db5868c)


