Utente:Grasso Luigi/sanbox1/Riarrangiamento Claisen
Il riarrangiamento di Claisen (da non confondere con la condensazione di Claisen) è una notevole reazione chimica di formazione del legame carbonio–carbonio scoperta da Rainer Ludwig Claisen. Il riscaldamento di un allil vinil etere innesca un [3,3]-riarrangiamento sigmatropico che produce un carbonile γ,δ-insaturo.

Scoperta nel 1912, la reazione di Claisen e' il primo esempio documentato di un [3,3]-riarrangiamento sigmatropico.[1][2][3] Sono state scritte molte recensioni.[4][5][6][7]
Meccanismo della reazione
[modifica | modifica wikitesto]Il riarrangiamento di Claisen è una reazione esotermica, ed è una reazione (legata al legame e alla ricombinazione) periciclica concertata. Le regole di Woodward-Hoffmann mostrano un percorso di reazione sovrafaciale, stereospecifico. La cinetica è del primo ordine e la trasformazione procede completamente attraverso uno stato ciclico di transizione altamente ordinato ed è un tipo di reazione intramolecolare.
Esperimenti di crossover eliminano la possibilità che il processo avvenga tramite un meccanismo di reazione intermolecolare e sono coerenti con un processo intramolecolare.[8][9]
There are substantial solvent effects observed in the Claisen rearrangement, where polar solvents tend to accelerate the reaction to a greater extent. Hydrogen-bonding solvents gave the highest rate constants. For example, ethanol/water solvent mixtures give rate constants 10-fold higher than sulfolane.[10][11] Trivalent organoaluminium reagents, such as trimethylaluminium, have been shown to accelerate this reaction.[12][13]
Variazioni
[modifica | modifica wikitesto]Riarrangiamento di Claisen aromatico
[modifica | modifica wikitesto]The first reported Claisen rearrangement is the [3,3]-sigmatropic rearrangement of an allyl phenyl ether to intermediate 1, which quickly tautomerizes to an ortho-substituted phenol.

Meta-substitution affects the regioselectivity of this rearrangement.[14][15] For example, electron withdrawing groups (e.g. bromide) at the meta-position direct the rearrangement to the ortho-position (71% ortho-product), while electron donating groups (e.g. methoxy), direct rearrangement to the para-position (69% para-product). Additionally, presence of ortho-substituents exclusively leads to para-substituted rearrangement products (tandem Claisen and Cope rearrangement).[16]
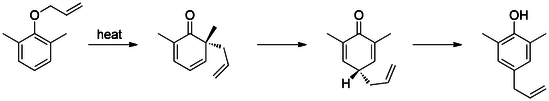
If an aldehyde or carboxylic acid occupies the ortho or para positions, the allyl side-chain displaces the group, releasing it as carbon monoxide or carbon dioxide, respectively.[17] [18]
Riarrangiamento di Bellus–Claisen
[modifica | modifica wikitesto]The Bellus–Claisen rearrangement is the reaction of allylic ethers, amines, and thioethers with ketenes to give γ,δ-unsaturated esters, amides, and thioesters.[19][20][21] This transformation was serendipitously observed by Bellus in 1979 through their synthesis of a key intermediate of an insecticide, pyrethroid. Halogen substituted ketenes (R1, R2) are often used in this reaction for their high electrophilicity. Numerous reductive methods for the removal of the resulting α-haloesters, amides and thioesters have been developed.[22] [23] The Bellus-Claisen offers synthetic chemists a unique opportunity for ring expansion strategies.

Riarrangiamento di Eschenmoser–Claisen
[modifica | modifica wikitesto]The Eschenmoser–Claisen rearrangement proceeds by heating allylic alcohols in the presence of N,N-dimethylacetamide dimethyl acetal to form γ,δ-unsaturated amide. This was developed by Albert Eschenmoser in 1964.[24][25] Eschenmoser-Claisen rearrangement was used as a key step in the total synthesis of morphine.[26]

Mechanism:[16]

Riarrangiamento di Ireland–Claisen
[modifica | modifica wikitesto]The Ireland–Claisen rearrangement is the reaction of an allylic carboxylate with a strong base (such as lithium diisopropylamide) to give a γ,δ-unsaturated carboxylic acid.[27][28][29]
The rearrangement proceeds via silylketene acetal, which is formed by trapping the lithium enolate with chlorotrimethylsilane. Like the Bellus-Claisen (above), Ireland-Claisen rearrangement can take place at room temperature and above. The E- and Z-configured silylketene acetals lead to anti and syn rearranged products, respectively.[30] There are numerous examples of enantioselective Ireland-Claisen rearrangements found in literature to include chiral boron reagents and the use of chiral auxiliaries.[31][32]

Riarrangiamento di Johnson–Claisen
[modifica | modifica wikitesto]The Johnson–Claisen rearrangement is the reaction of an allylic alcohol with an orthoester to yield a γ,δ-unsaturated ester. [33] Weak acids, such as propionic acid, have been used to catalyze this reaction. This rearrangement often requires high temperatures (100 to 200 °C) and can take anywhere from 10 to 120 hours to complete.[34] However, microwave assisted heating in the presence of KSF-clay or propionic acid have demonstrated dramatic increases in reaction rate and yields.[35][36]

Mechanism:[16]

Riarrangiamento foto-Claisen
[modifica | modifica wikitesto]The photo-Claisen rearrangement is closely related to the photo-Fries rearrangement, that proceeds through a similar radical mechanism. Aryl ethers undergo the photo-Claisen rearrangement, while the photo-Fries rearrangement utilizes aryl esters.[37]
Eteroriarrangiamenti
[modifica | modifica wikitesto]Riarrangiamento di Aza–Claisen
[modifica | modifica wikitesto]An iminium can serve as one of the pi-bonded moieties in the rearrangement.[38]

Ossidazione del cromo
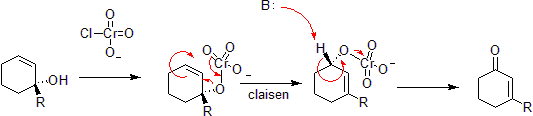
[modifica | modifica wikitesto]Chromium can oxidize allylic alcohols to alpha-beta unsaturated ketones on the opposite side of the unsaturated bond from the alcohol. This is via a concerted hetero-Claisen reaction, although there are mechanistic differences since the chromium atom has access to d- shell orbitals which allow the reaction under a less constrained set of geometries.[39] [40]
Reazione di Chen–Mapp
[modifica | modifica wikitesto]The Chen–Mapp reaction also known as the [3,3]-Phosphorimidate Rearrangement or Staudinger–Claisen Reaction installs a phosphite in the place of an alcohol and takes advantage of the Staudinger reduction to convert this to an imine. The subsequent Claisen is driven by the fact that a P=O double bond is more energetically favorable than a P=N double bond.[41]

Riarrangiamento di Overman
[modifica | modifica wikitesto]The Overman rearrangement (named after Larry Overman) is a Claisen rearrangement of allylic trichloroacetimidates to allylic trichloroacetamides.[42][43][44]

Overman rearrangement is applicable to synthesis of vicinol diamino comp from 1,2 vicinal allylic diol.
Riarrangiamento zwitterionico di Claisen
[modifica | modifica wikitesto]Unlike typical Claisen rearrangements which require heating, zwitterionic Claisen rearrangements take place at or below room temperature. The acyl ammonium ions are highly selective for Z-enolates under mild conditions.[45][46]

Reazione di Claisen naturale
[modifica | modifica wikitesto]The enzyme chorismate mutase (EC 5.4.99.5) catalyzes the Claisen rearrangement of chorismate to prephenate, a key intermediate in the shikimate pathway (the biosynthetic pathway towards the synthesis of phenylalanine and tyrosine).[47]

Voci correlate
[modifica | modifica wikitesto]Note
[modifica | modifica wikitesto]- ^ (EN) Claisen, L., Über Umlagerung von Phenol-allyläthern in C-Allyl-phenole, in Chem. Ber., vol. 45, n. 3, 1912, pp. 3157–3166, DOI:10.1002/cber.19120450348.
- ^ (EN) Claisen, L. e Tietze, E., Über den Mechanismus der Umlagerung der Phenol-allyläther, in Chemische Berichte, vol. 58, 1925, p. 275, DOI:10.1002/cber.19250580207.
- ^ (EN) Claisen, L. e Tietze, E., Über den Mechanismus der Umlagerung der Phenol-allyläther. (2. Mitteilung), in Chemische Berichte, vol. 59, 1926, p. 2344, DOI:10.1002/cber.19260590927.
- ^ {(EN) Hiersemann, M. e Nubbemeyer, U., The Claisen Rearrangement, Wiley-VCH, 2007, ISBN 3-527-30825-3.
- ^ (EN) Rhoads, S. J.; Raulins, N. R., The Claisen and Cope Rearrangements, in Org. React., vol. 22, 1975, pp. 1–252, DOI:10.1002/0471264180.or022.01, ISBN 0471264180.
- ^ (EN) Ziegler, F. E., The thermal, aliphatic Claisen rearrangement, in Chem. Rev., vol. 88, n. 8, 1988, pp. 1423–1452, DOI:10.1021/cr00090a001.
- ^ (EN) Wipf, P., Claisen Rearrangements, in Compr. Org. Synth., vol. 5, 1991, pp. 827–873, DOI:10.1016/B978-0-08-052349-1.00140-2, ISBN 978-0-08-052349-1.
- ^ (EN) Hurd, C. D.; Schmerling, L., Observations on the Rearrangement of Allyl Aryl Ethers, in J. Am. Chem. Soc., vol. 59, 1937, p. 107, DOI:10.1021/ja01280a024.
- ^ {(EN) Francis A. Carey;author2=Richard J. Sundberg, 10, in Advanced Organic Chemistry: Part A: Structure and Mechanisms, 5ed, Berlino, Springer, 2007, pp. 934–935, DOI:10.1007/978-0-387-44899-2, ISBN 978-0-387-44897-8 (Print).
- ^ Claisen, L., Über Umlagerung von Phenol-allyläthern in C-Allyl-phenole, in Chemische Berichte, vol. 45, n. 3, 1912, pp. 3157–3166, DOI:10.1002/cber.19120450348.
- ^ Über den Mechanismus der Umlagerung der Phenol-allyläther, in Chemische Berichte, vol. 58, n. 2, 1925, DOI:10.1002/cber.19250580207.
- ^ A Kinetic Study of the ortho-Claisen Rearrangement1, in J. Am. Chem. Soc., vol. 80, n. 13, 1958, DOI:10.1021/ja01546a024.
- ^ The o-Claisen rearrangement. VIII. Solvent effects, in J. Org. Chem., vol. 35, n. 7, 1970, DOI:10.1021/jo00832a019.
- ^ White, William; and Slater, Carl, The ortho-Claisen Rearrangement. V. The Products of Rearrangement of Allyl m-X-Phenyl Ethers, in The Journal of Organic Chemistry, vol. 26, n. 10, 1961, pp. 3631–3638, DOI:10.1021/jo01068a004.
- ^ Gozzo, Fábio; Fernandes, Sergio; Rodrigues, Denise; Eberlin, Marcos; and Marsaioli, Anita, Regioselectivity in Aromatic Claisen Rearrangements, in The Journal of Organic Chemistry, vol. 68, n. 14, 2003, pp. 5493–5499, DOI:10.1021/jo026385g.
- ^ a b c Strategic Applications Of Named Reactions In Organic Synthesis: Background And Detailed Mechanics: 250 Named Reactions, Academic Press, 2005, ISBN 978-0-12-429785-2. URL consultato il 27 March 2013.
- ^ Rodger Adams, Organic Reactions, Volume II, Newyork, John Wiley & Sons, Inc., 1944, pp. 11–12.
- ^ L. Claisen, Über die Umlagerung von Phenolallyläthern in die isomeren Allylphenole, in Justus Liebigs Annalen der Chemie, vol. 401, n. 1, 1913, DOI:10.1002/jlac.19134010103.
- ^ A New Type of Claisen Rearrangement Involving 1,3-Dipolar Intermediates. Preliminary communication, in Helv. Chim. Acta, vol. 61, n. 8, 1978, pp. 3096–3099, DOI:10.1002/hlca.19780610836.
- ^ Reactions of haloketenes with allyl ethers and thioethers: A new type of Claisen rearrangement, in J. Org. Chem., vol. 48, n. 6, 1983, pp. 860–869, DOI:10.1021/jo00154a023.
- ^ Gonda, J., The Belluš–Claisen Rearrangement, in Angew. Chem. Int. Ed., vol. 43, n. 27, 2004, pp. 3516–3524, DOI:10.1002/anie.200301718.
- ^ E Edstrom, An unexpected reversal in the stereochemistry of transannular cyclizations. A stereoselective synthesis of (±)-epilupinine., in Tetrahedron Letters, 1991, DOI:10.1016/S0040-4039(00)93536-6.
- ^ Bellus, Reactions of haloketenes with allyl ethers and thioethers: a new type of Claisen rearrangement, in JOC, 1983, DOI:10.1021/jo00154a023.
- ^ CLAISEN'sche Umlagerungen bei Allyl- und Benzylalkoholen mit Hilfe von Acetalen des N, N-Dimethylacetamids. Vorläufige Mitteilung, in Helv. Chim. Acta, vol. 47, n. 8, 1964, pp. 2425–2429, DOI:10.1002/hlca.19640470835.
- ^ CLAISEN'sche Umlagerungen bei Allyl- und Benzylalkoholen mit 1-Dimethylamino-1-methoxy-äthen, in Helv. Chim. Acta, vol. 52, n. 4, 1969, pp. 1030–1042, DOI:10.1002/hlca.19690520418.
- ^ C Guillou, Diastereoselective Total Synthesis of (±)-Codeine, in Chem. Eur. J., 2008, DOI:10.1002/chem.200800744.
- ^ Claisen rearrangement of allyl esters, in JACS, vol. 94, n. 16, 1972, DOI:10.1021/ja00771a062.
- ^ The stereoselective generation of ester enolates, in Tetrahedron Lett., vol. 16, n. 46, 1975, pp. 3975–3978, DOI:10.1016/S0040-4039(00)91213-9.
- ^ The ester enolate Claisen rearrangement. Stereochemical control through stereoselective enolate formation, in JACS, vol. 98, n. 10, 1976, DOI:10.1021/ja00426a033.
- ^ R. E. Ireland, Stereochemical control in the ester enolate Claisen rearrangement., in JOC, 1991, DOI:10.1021/jo00002a030.
- ^ E Enders, Asymmetric [3.3]-sigmatropic rearrangements in organic synthesis, in Tetrahedron: Asymmetry, 1996, DOI:10.1016/0957-4166(96)00220-0.
- ^ E Corey, Highly enantioselective and diastereoselective Ireland-Claisen rearrangement of achiral allylic esters, in JACS, 1991, DOI:10.1021/ja00010a074.
- ^ William Summer Johnson, Simple stereoselective version of the Claisen rearrangement leading to trans-trisubstituted olefinic bonds. Synthesis of squalene, in JACS, vol. 92, n. 3, 1º febbraio 1970, pp. 741–743, DOI:10.1021/ja00706a074.
- ^ R. A. Fernandes, The Orthoester Johnson–Claisen Rearrangement in the Synthesis of Bioactive Molecules, Natural Products, and Synthetic Intermediates – Recent Advances, in Eur JOC, 2013, DOI:10.1002/ejoc.201301033.
- ^ R. S. Huber, Acceleration of the orthoester Claisen rearrangement by clay catalyzed microwave thermolysis: expeditious route to bicyclic lactones, in JOC, 1992, DOI:10.1021/jo00047a041.
- ^ A Srikrishna, Application of microwave heating technique for rapid synthesis of γ,δ-unsaturated esters, in Tetrahedron, 1995, DOI:10.1016/0040-4020(94)01058-8.
- ^ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006–) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8. DOI: 10.1351/goldbook
- ^ Enantioselective preparation of 3-substituted 4-pentenoic acids via the Claisen rearrangement, in J. Org. Chem., vol. 50, n. 26, 1985, pp. 5769–5775, DOI:10.1021/jo00350a067.
- ^ Direct oxidation of tertiary allylic alcohols. A simple and effective method for alkylative carbonyl transposition, in J. Org. Chem., vol. 42, n. 4, 1977, DOI:10.1021/jo00424a023.
- ^ (R)-(+)-3,4-DIMETHYLCYCLOHEX-2-EN-1-ONE [(R)-(+)-3,4-Dimethyl-2-cyclohexen-1-one], su orgsyn.org.
- ^ Thermal and catalyzed 3,3-phosphorimidate rearrangements, in JACS, vol. 127, n. 18, 2005, pp. 6712–6718, DOI:10.1021/ja050759g.
- ^ Thermal and mercuric ion catalyzed [3,3]-sigmatropic rearrangement of allylic trichloroacetimidates. 1,3 Transposition of alcohol and amine functions, in JACS, vol. 96, n. 2, 1974, pp. 597–599, DOI:10.1021/ja00809a054.
- ^ A general method for the synthesis of amines by the rearrangement of allylic trichloroacetimidates. 1,3 Transposition of alcohol and amine functions, in JACS, vol. 98, n. 10, 1976, pp. 2901–2910, DOI:10.1021/ja00426a038.
- ^ Organic Syntheses, Coll. Vol. 6, p.507; Vol. 58, p.4 (Article)
- ^ Self-regulated molecular rearrangement: Diastereoselective zwitterionic aza-Claisen protocol, in J. Chem. Soc., Perkin Trans. 1, n. 2, 1996, pp. 115–116, DOI:10.1039/p19960000115.
- ^ Nubbemeyer, U., 1,2-Asymmetric Induction in the Zwitterionic Claisen Rearrangement of Allylamines, in JOC, vol. 60, n. 12, 1995, pp. 3773–3780, DOI:10.1021/jo00117a032.
- ^ Ganem, B., The Mechanism of the Claisen Rearrangement: Déjà Vu All over Again, in Angew. Chem. Int. Ed. Engl., vol. 35, n. 9, 1996, pp. 936–945, DOI:10.1002/anie.199609361.
Altri progetti
[modifica | modifica wikitesto]