Utente:Grasso Luigi/sanbox1/Fosfenio
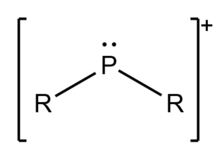
Lo ione fosfenio, da non confondere con fosfonio o fosfirenio, è un catione divalente o dicatione formato da fosforo di struttura chimica [PR2]+. Phosphenium ions have long been proposed as reaction intermediates, but the first examples of cyclic phosphenium compounds weren't prepared until 1972 by Suzanne Fleming and coworkers.[1] The first acyclic phosphenium compounds were synthesized by Robert Parry and coworkers a few years later in 1976.[2]

Sintesi[modifica | modifica wikitesto]
Phosphorus-Halogen bond heterolysis[modifica | modifica wikitesto]
There are several methods for preparation of two-coordinate phosphorus ions, but the most common method is by phosphorus-halogen bond heterolysis. Typically, chloride ions are abstracted from a chlorophosphine, but phosphenium ions have also been prepared from the heterolysis of P-F bonds.[3] The reaction is accomplished by warming from low, but not cryogenic, temperatures (eg. -78 °C).

Phosphorus-Nitrogen bond protonolysis[modifica | modifica wikitesto]
A less popular and more challenging route for phosphenium synthesis is via P-N bond protonolysis. Protonation of the amide nitrogen weakens the P-N bond, resulting in bond cleavage. This method may result in several undesirable side products, such as complexation of the phosphenium with the conjugate base yielding an adduct, or the protonation of the Phosphorus atom resulting in the four coordinate aminophosphonium product.[4] Weakly coordinating conjugate bases (A) are desirable and potentially limit adduct formation with phosphenium. Triflic acid is often used.[3]

N-heterocyclic phosphenium redox synthesis[modifica | modifica wikitesto]
Recently, the facile, one-step synthesis of N-heterocyclic phosphenium (NHP) has been reported.[5] Reaction of PI3 with the desired α-diimine yields the NHP cation by reduction of the diimine and oxidation of Iodine.

Struttura[modifica | modifica wikitesto]
X-ray crystallography of [(i-Pr2N)2P]+ revealed the structure of the ion as a nearly planar molecule with an approximately sp2 hybridized phosphorus center.[6] The planarity of the nitrogen center is consistent with the resonance of the lone pair of the nitrogen atom as a pi bond to the empty phosphorus 3p orbital perpendicular to the N-P-N plane. An idealized sp2 phosphorus center would expect an N-P-N angle of 120°. The tighter N-P-N angle observed in the crystal structure can be interpreted as the result of repulsion between the phosphorus lone pair with the bulky i-Pr2N ligands, as the P(NH2)2+ and PH2+ molecules have bond angles closer to 110° and 90°, respectively.[3][6]

Reattività[modifica | modifica wikitesto]
Addotti base-acido di Lewis[modifica | modifica wikitesto]
Phosphenium is isoelectronic with singlet (Fisher) carbenes and are therefore predicted to behave as both a Lewis acid and a Lewis base. The Lewis acidity of phosphenium is evident from the reaction of model phosphenium ions, such as [P(NMe2)2]+ and [P(NMe2)Cl]+ with a Lewis base such as P(NMe2)3. Such a reaction results in the formation of the expected adducts.[2] Additional Lewis adducts have since been characterized, including adducts of P(NMe2)(t-Bu)(F) and [P(NMe2)(t-Bu)]+ as well as P(t-Bu)3 and [P(Fc)2]+ (Fc = ferrocene). [7][8]

Reazioni con inserzione di C-H[modifica | modifica wikitesto]
Some of the first C-H insertion reactions utilizing phosphenium ions were characterized by Alan Cowley. During the attempted formation of P-E multiple bonds (where E = Sn, Pb), it was observed that the phosphenium ion inserted into a C-H bond of stannocene or plumbocene, either through oxidative addition or electrophilic aromatic substitution, to generate a phosphonium ion.[9] Following this initial discovery, several other examples of C-H bond activation have been reported.[10][11]

Reazioni con dieni[modifica | modifica wikitesto]

In a manner similar to Diels-Alder cycloaddition reactions, phosphenium ions have been demonstrated to react with 1,3-dienes by 1,4-addition.[13][14][15][3] As the mechanism for "Diels-Alder-like" cycloadditions is still the subject of debate, the mechanism of these reactions with phosphenium are not well known. Carbenes are known to react via a [2+2] chelotropic cycloaddition, followed by rearrangement to the observed product. However, the sterioselectivity of the reaction supports a single-step [2+4] chelotropic cycloaddition directly to product.[3][12]

Catalisi[modifica | modifica wikitesto]
There are few examples of reactions catalyzed by phosphenium. In 2018, Rei Kinjo and coworkers reported the hydroboration of pyridines by the NHP salt, 1,3,2-diazaphosphenium triflate. The NHP is proposed to act as a hydride transfer reagent in this reaction.[16]

Chimica di coordinazione[modifica | modifica wikitesto]
Phosphenium ions have similar electronic structure to singlet (Fisher) carbenes, and it is therefore anticipated that they would have similar behavior as ligands in coordination chemistry. The first phosphenium ligated transition metal complexes where prepared by Robert Parry.[2] [(R2N)2PFe(CO)4]+ was prepared by two methods: the first being the abstraction of a fluoride ion from (R2N)2(F)PFe(CO)4 by PF5. The second method is the direction substitution reaction of Fe(CO)5 by the phosphenium ion [P(NR2)]+. [17] In the time since this initial report several phosphenium ions have been complexed with Fe(CO)4, including [(Me2N)2P]+, [(Et2N)2P]+, [(Me2N)(Cl)P]+, and [(en)P]+ (en = C2H4(NH2)2).[3]

Several examples of molybdenum-phosphenium complexes also exist. The mononuclear Mo complex Mo(CO)2CpP(NMe)2C2H4 was first synthesized by Robert Paine from [CpMo(CO)3] and cyclic diaminohalophosphine.[18][19] The dinuclear complex was also prepared by Paine in 1983.[20] The full list of Mo-phosphenium complexes is extensive and a more detailed account can be found in refs.[21] and [3].

N-heterocyclic phosphenium-transition metal complexes are anticipated due to their isoelectronicity to N-heterocyclic carbenes. In 2004, Martin Nieger and coworkers synthesized two Cobalt-NHP complexes. Experimental and computation analysis of the complexes confirmed the expected L→M σ donation and the M→L π backbonding, though the phosphenium was observed to have reduced σ donor ability. It was suggested that this is due to the greater s orbital-character of the phosphorus lone pair compared to the lone pair of the analogous carbene.[22] Additional studies of NHP ligands by Christine Thomas and coworkers in 2012, likened the phosphenium to nitrosyl.[23] Nitrosyl is well known for its redox non-innocence, coordinating in either a bent or linear geometry that possess different L-M bonding modes. It was observed that NHPs in complex with a transition metal may have either a planar or pyramidal geometry about the phosphorus, reminiscent of the linear versus bent geometries of nitrosyl. Highly electron-rich metal complexes were observed to have pyramidal phosphorus, while less electron-rich metals showed greater phosphenium character at the phosphorus. Pyramidal phosphorus indicates significant lone pair character at phosphorus, suggesting that the L→M σ donation and the M→L π backbonding interactions have been replaced with M→L σ donation, formally oxidizing the metal center by two electrons.[23]

Calcoli teorici[modifica | modifica wikitesto]
Calculated geometries of the model phosphenium ions [PH2]+, [PF2]+, and [P(NH2)2]+ agree with the observed geometries. Density functional theory (DFT) calculations of the model compounds using the B3LYP 6-31G** basis set yielded orbital diagrams that agree with the expectations that the lone pair of the phosphorus lies in the R-P-R plane, and that the lowest unoccupied molecular orbital (LUMO) is the phosphorus p-orbital directed perpendicular to the molecular plane. As noted above, the observed geometry at the nitrogen atoms in [P(NH2)2]+ and related compounds is trigonal planar, implicating a degree of resonance stabilization via pi-donation from the occupied nitrogen p-orbitals to the empty phosphorus p-orbital. Simulation of the model compound [P(NH2)2]+ is in agreement with this observation: the LUMO of the ion is the antibonding conjugate of the lower lying pi-bonding orbital. Additionally, with strongly donating substituents, such as NH2, the highest occupied molecular orbital (HOMO) is the antisymmetric combination of the nitrogen p-orbitals, and the lone pair of the phosphorus are below them in energy (HOMO-1). These calculations also show that the analogy to carbenes is lessened by strongly π-donating substituents, such as NH2, as the phosphenium now has some allyl character.[24]
| PH2+ | PF2+ | P(NH2)2+ | |
|---|---|---|---|
HOMO |
HOMO |
HOMO |
HOMO-1 |
LUMO |
LUMO |
LUMO | |
Generalized Valence Bond (GVB) calculations of the phosphenium ions as having a singlet ground state, singlet-triplet separation increases with increasing electronegativity of the ligands.[3][25][26] The singlet-triplet separation for PH2+ and PF2+ were calculated to be 20.38 and 84.00 kcal/mol, respectively. Additionally, the triplet state of the phosphenium ion displays a greater bond angle at the phosphorus. For example, the calculated bond angle of the singlet state of PH2+ is approximately 94° compared to 121.5° in the triplet state. Calculated bond lengths between the two states are not significantly impacted.[26]
| Molecule | state | Energy (hartrees) |
|---|---|---|
| PH2+ | 1A1 | -341.562170 |
| PH2+ | 3B1 | -341.529685 |
| PF2+ | 1A1 | -539.422552 |
| PF2+ | 3B1 | -539.288625 |
Note[modifica | modifica wikitesto]
- ^ a b (EN) Suzanne. Fleming, Synthesis of a cyclic fluorodialkylaminophosphine and its coordination with boron acids. Formation of a unique dialkylaminophosphine cation, in Inorganic Chemistry, vol. 11, n. 10, 1º ottobre 1972, pp. 2534–2540, DOI:10.1021/ic50116a050.
- ^ a b c d e (EN) C. W. Schultz, Structure of [2((CH3)2N)2PCl].AlCl3, ((CH3)2N)3P.((CH3)2N)2PCl.AlCl3, and related species-diphosphorus cations, in Inorganic Chemistry, vol. 15, n. 12, 1º dicembre 1976, pp. 3046–3050, DOI:10.1021/ic50166a022.
- ^ a b c d e f g h i j k l m n o (EN) A. H. Cowley, Synthesis and reaction chemistry of stable two-coordinate phosphorus cations (phosphenium ions), in Chemical Reviews, vol. 85, n. 5, 1º ottobre 1985, pp. 367–382, DOI:10.1021/cr00069a002.
- ^ Otto Dahl, Reactions of aminophosphines with trifluormethanesulfonic acid: phosphenium ion (two-coordinate phosphorus ion) or tricovalent phosphorus products?, in Tetrahedron Letters, vol. 23, n. 14, 1º gennaio 1982, pp. 1493–1496, DOI:10.1016/s0040-4039(00)87141-5.
- ^ a b (EN) Gregor Reeske, One-Step Redox Route to N-Heterocyclic Phosphenium Ions, in Inorganic Chemistry, vol. 46, n. 4, 1º febbraio 2007, pp. 1426–1430, DOI:10.1021/ic061956z.
- ^ a b c (EN) Alan H. Cowley, Static and dynamic stereochemistry of dicoordinate phosphorus cations, in Journal of the American Chemical Society, vol. 100, n. 24, 1º novembre 1978, pp. 7784–7786, DOI:10.1021/ja00492a087.
- ^ (EN) S. G. Baxter, Ferrocenyl-substituted phosphenium cations and phosphide anions, in Inorganic Chemistry, vol. 22, n. 23, 1º novembre 1983, pp. 3475–3479, DOI:10.1021/ic00165a022.
- ^ (EN) A. H. Cowley, NMR study of the reactions of phosphorus(III) halides with halide ion acceptors. Two-coordinate phosphorus cations with bulky ligands, in Inorganic Chemistry, vol. 20, n. 9, 1º settembre 1981, pp. 2916–2919, DOI:10.1021/ic50223a034.
- ^ a b (EN) A. H. Cowley, Reaction of stannocene and plumbocene with phosphenium ions: oxidative addition of carbon-hydrogen bonds to low-coordination number main group species, in Journal of the American Chemical Society, vol. 104, n. 11, 1º giugno 1982, pp. 3239–3240, DOI:10.1021/ja00375a061.
- ^ (EN) A. H. Cowley, Ring methyl to phosphorus hydrogen shifts in pentamethylcyclopentadienyl-substituted phosphorus cations: parallel between main-group and transition-metal chemistry, in Journal of the American Chemical Society, vol. 105, n. 7, 1º aprile 1983, pp. 2074–2075, DOI:10.1021/ja00345a072.
- ^ (EN) Hiroshi Nakazawa, Reactions of phosphorus electrophiles with [(.eta.5-C5Me5)Fe(CO)2]-; spectroscopic evidence for a phosphinidene complex, in Inorganic Chemistry, vol. 23, n. 22, 1º ottobre 1984, pp. 3431–3433, DOI:10.1021/ic00190a001.
- ^ a b A. H. Cowley, Reactivity of phosphenium ions toward 1,3- and 1,4-dienes, in Inorganic Chemistry, vol. 25, n. 6, 1º marzo 1986, pp. 740–749, DOI:10.1021/ic00226a007.
- ^ (EN) Carlton K. SooHoo, Phosphenium ions as dienophiles, in Journal of the American Chemical Society, vol. 105, n. 25, 1º novembre 1983, pp. 7443–7444, DOI:10.1021/ja00363a039.
- ^ (EN) A. H. Cowley, Reaction of phosphenium ions with 1,3-dienes: a rapid synthesis of phosphorus-containing five-membered rings, in Journal of the American Chemical Society, vol. 105, n. 25, 1º novembre 1983, pp. 7444–7445, DOI:10.1021/ja00363a040.
- ^ (EN) Michael G. Thomas, Synthesis and characterization of dicoordinate phosphorus cations. Compounds of the type [(R2N)2P]+[Y]- and their congeners, in Inorganic Chemistry, vol. 16, n. 5, 1º maggio 1977, pp. 994–1001, DOI:10.1021/ic50171a005.
- ^ a b (EN) Bin Rao, Metal-Free Regio- and Chemoselective Hydroboration of Pyridines Catalyzed by 1,3,2-Diazaphosphenium Triflate, in Journal of the American Chemical Society, vol. 140, n. 2, 5 gennaio 2018, pp. 652–656, DOI:10.1021/jacs.7b09754.
- ^ a b (EN) R. G. Montemayor, Iron carbonyl complexes containing positively charged phosphorus ligands, in Journal of the American Chemical Society, vol. 100, n. 7, 1º marzo 1978, pp. 2231–2233, DOI:10.1021/ja00475a044.
- ^ a b (EN) L. D. Hutchins, Structure and bonding in a phosphenium ion-metal complex, CH3NCH2CH2N(CH3)PMo(.eta.5-C5H5)(CO)2. An example of a molybdenum-phosphorus multiple bond, in Journal of the American Chemical Society, vol. 102, n. 13, 1º giugno 1980, pp. 4521–4523, DOI:10.1021/ja00533a039.
- ^ a b (EN) R. W. Light, Interaction of the dicoordinate phosphorus cation 1,3-dimethyl-1,3,2-diazaphospholidide with transition metal nucleophiles, in Journal of the American Chemical Society, vol. 100, n. 7, 1º marzo 1978, pp. 2230–2231, DOI:10.1021/ja00475a043.
- ^ (EN) Donn A. Dubois, Synthesis and structure of a bimetallic diphosphenium ion complex containing a diazadiphosphetidine ring, in Organometallics, vol. 2, n. 12, 1º dicembre 1983, pp. 1903–1905, DOI:10.1021/om50006a044.
- ^ (EN) Larry D. Hutchins, Synthesis and characterization of metallophosphenium ion complexes derived from aminohalophosphites. Crystal and molecular structure of [cyclo]Mo(.eta.5-C5H5)(CO)2(POCH2CH2NCMe3), in Organometallics, vol. 3, n. 3, 1º marzo 1984, pp. 399–403, DOI:10.1021/om00081a013.
- ^ a b (EN) Sebastian Burck, N-Heterocyclic Phosphenium, Arsenium, and Stibenium Ions as Ligands in Transition Metal Complexes: A Comparative Experimental and Computational Study, in Zeitschrift für Anorganische und Allgemeine Chemie, vol. 631, n. 8, 1º giugno 2005, pp. 1403–1412, DOI:10.1002/zaac.200400538.
- ^ a b (EN) Baofei Pan, N-Heterocyclic Phosphenium Ligands as Sterically and Electronically-Tunable Isolobal Analogues of Nitrosyls, in Inorganic Chemistry, vol. 51, n. 7, 14 marzo 2012, pp. 4170–4179, DOI:10.1021/ic202581v.
- ^ a b (EN) Dietrich Gudat, <1087::aid-ejic1087>3.0.co;2-3 Cation Stabilities, Electrophilicities, and "Carbene Analogue" Character of Low Coordinate Phosphorus Cations, in European Journal of Inorganic Chemistry, vol. 1998, n. 8, 1º agosto 1998, pp. 1087–1094, DOI:10.1002/(sici)1099-0682(199808)1998:8<1087::aid-ejic1087>3.0.co;2-3.
- ^ James F. Harrison, The multiplicity of substituted acyclic carbenes and related molecules, in Journal of the American Chemical Society, vol. 101, n. 24, 1º novembre 1979, pp. 7162–7168, DOI:10.1021/ja00518a006.
- ^ a b c (EN) James F. Harrison, Electronic structure of the phosphenium ions PH2+, HPF+, and PF2+, in Journal of the American Chemical Society, vol. 103, n. 25, 1º dicembre 1981, pp. 7406–7413, DOI:10.1021/ja00415a002.
Voci correlate[modifica | modifica wikitesto]
Altri progetti[modifica | modifica wikitesto]